CH4, or methane, is a natural, colorless, and flammable gas. It’s made up of one carbon atom and four hydrogen atoms. The molecular geometry of CH4 is Tetrahedral. It is an alkane and also the simplest saturated hydrocarbon. Methane is one of the most important greenhouse gases. About 70% of the total emissions of methane are due to human activities.
It’s used for various domestic and commercial purposes as it’s a very efficient source of energy.
Before further explanation, let’s enlist the few important properties of CH4:
- Methane is insoluble in water, but it’s soluble in ethanol, ether, and benzene.
- It’s easily ignitable.
Further Properties Are Listed In The Table Below:
| Name | Methane |
| Chemical formula | CH4 |
| Molecular geometry | Tetrahedral |
| Boiling point | -161.5 oC |
| Melting point | -182.45 oC |
| Bond angle | 109.5o |
| Molar mass | 16.043gmol-1 |
| Electron geometry | Tetrahedral |
| Valence electrons | 8 |
| Hybridization | Sp3 |
| Density | 0.656kgm-3 |
| Charge/polarity | 0 |
In this article, you will come to know about the following points:
- Molecular geometry of CH4.
- CH4 Lewis dot structure.
- Hybridization of CH4 molecule.
- Orbital geometry of CH4.
- Polarity and non-polarity of CH4.
- CH4 bond angle and type.
- Valence electrons in CH4.
- Different uses of CH4.
Now let’s discuss the headings one by one.
Molecular Geometry Of CH4:
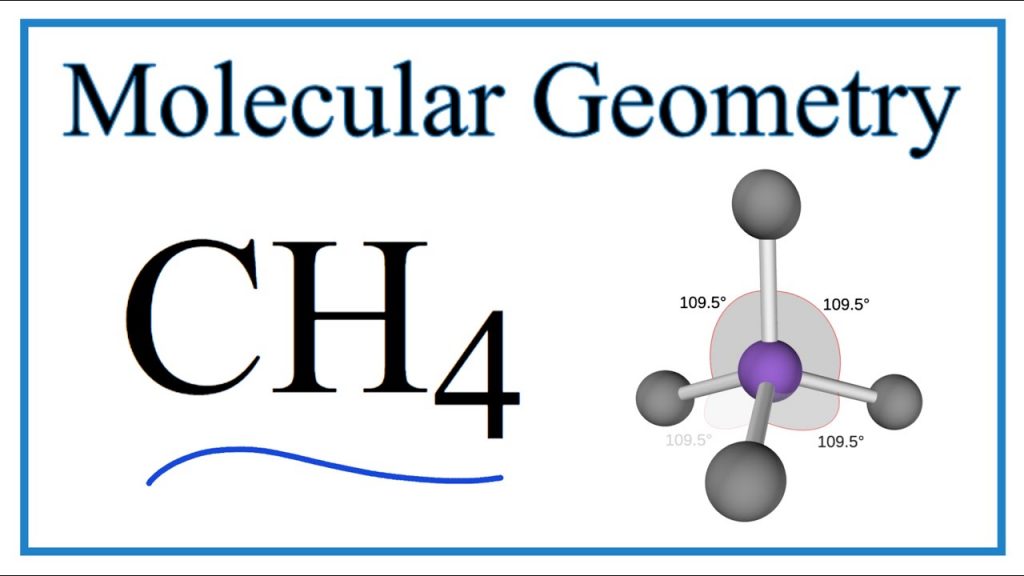
The molecular geometry/shape of CH4 is tetrahedral.
As you can see in the above figure, carbon is the central atom in the tetrahedron and four hydrogen atoms are located on the four sides. As the four hydrogen atoms are carrying the same charge so they repel each other in a way that the final tetrahedron shape is formed.
The four hydrogen atoms are attached by covalent bonds because the central carbon atom doesn’t share a lone pair of electrons.
The positions of the four hydrogen atoms in the CH4 molecule are the point where the repulsion of similar charges is minimum. And the same is for every tetrahedral molecule.
In the above figure, the green lines denote the tetrahedral shape and the black lines denote the bonds between hydrogen and carbon also showing the bond angle which is 109.5o.
If you have heard about VSEPR theory, it also states that electron pairs of the same nature repel each other until the repulsion becomes minimum and the molecule attains a tetrahedral geometry.
Octet Rule Regarding CH4:
The octet rule states that an atom needs a maximum of eight electrons in its valence shell to attain maximum stability.
In CH4 carbon has four valence electrons and four hydrogen atoms have a total of four valence electrons. Thus, making a total of eight valence electrons for the molecule and making it a stable and non-polar molecule.
We will further discuss this octet rule in the Lewis dot structure section below with the help of a diagram:
CH4 Lewis Dot Structure:
We can say that Lewis’s structure is the pictorial representation of the arrangement of electrons present in the valence shell of any atom.
These electrons determine the bond type, bond angle, and geometrical symmetry of a molecule.
Let me explain to you the Lewis dot structure of methane with the help of a diagram.
As you can see in the figure above, the Lewis structure is made up of a single central atom and four hydrogen atoms present on the four sides. The dots show a number of electrons and the lines show the bond between the two atoms.
Lewis’s structure works according to the octet rule which states that an atom needs eight electrons in its valence shell to become stable.
In the case of CH4, each hydrogen atom needs only one electron to complete its octet. So, it shares the valence electron of carbon and makes a single covalent bond. As carbon has four valence electrons, it shares all of them with four hydrogen atoms to make four bonding pairs of electrons and no loan pair is visible in the structure.
Carbon occupies the central position due to its less electronegativity than hydrogen.
Hybridization Of CH4 Molecule:
Hybridization we can say is the mathematical process in which we study the mixing and overlapping of a minimum of two atomic orbitals of the same atom to produce a new orbital having the same energy.
If we consider the hybridization of CH4, it’s Sp3. The reason behind it is that there are one 2s and three 2p orbitals of carbon present which mix and overlap to form four new orbitals of the same energy as we discussed earlier in the topic.
The new Sp3 orbitals have 25% characteristics of S orbitals ad 75% characteristics of P orbitals because they are greater in number.
Read More: The Complete Guide To Snapchat Unlock
Sigma bonds are formed between hydrogen and carbon (C-H) in methane with the help of the newly formed hybrid orbitals by four hydrogen atoms.
As it’s explained in the figure, there is no pi (π) bond present in the CH4 molecule but one sigma (σ) bond exists. Similarly, if one sigma bond is present between one hydrogen and one carbon atom then four sigma bonds are present between four hydrogen atoms and one carbon atom as carbon has four valence electrons.
These sigma bonds determine the hybridization of the CH4 molecule.
Orbital Geometry Of CH4:
Orbital geometry is the term that is often used to explain the hybridization of a molecule. It tells us how the overlapping is taking place.
The Polarity Of Molecule:
The polarity of the molecule depends upon its net dipole moment. If a molecule has an unequal distribution of charges, it must have some dipole moment and is a polar one. But if there is equal distribution of charges the net dipole moment is zero because equal charges cancel each other.
Is CH4 Polar Or Non-Polar?
CH4 is a non-polar molecule because it is made up of four similar C-H bonds which are arranged on the four corners of a central atom in a tetrahedral geometry. Due to this type of arrangement, the net dipole moment becomes zero because it is canceled. Because similar bonds are present on each side.
There are certain factors upon which the polarity of the molecule depends. These are:
1. Electronegativity:
First and the most important factor determining the polarity of any molecule is the electronegativity difference between atoms of that molecule.
It directly belongs to polarity. If there is a greater electronegativity difference then the molecule is highly polar and vice versa.
In addition to this, according to the Pauling scale if the electronegativity difference between atoms is less than 0.5 then the molecule or bond is non-polar. In CH4, the electronegativity of carbon is 2.6 and that of hydrogen is 2.2. The difference between the electronegativities of the two atoms is 0.4 (<0.5) so CH4 is non-polar.
If you think, CH4 becomes polar due to the electronegativity difference between C-H then you are not right. Because this difference is canceled due to the tetrahedral geometry of CH4.
2. Molecular Geometry:
The symmetry/shape of a molecule also plays a role in determining its polarity.
As you know, the geometry of the CH4 molecule is tetrahedral. For non-polar covalent bonds are present in opposite directions. The central carbon atom doesn’t contain any loan pair so the tetrahedron becomes more symmetrical.
These highly symmetrical covalent bonds (C-H) cancel each other making CH4 an overall non-polar molecule.
Read More: The Complete Guide To Trust Browser Enable
3. Dipole Moment:
The difference between the electronegativities of different atoms determines the dipole moment.
Because of the electronegativity difference between carbon and hydrogen atoms, some negative charge is produced on the carbon atom and a positive charge on the hydrogen atom. These opposite charges create four dipole moments forming C-H bonds.
As you can see in the figure below, four dipole moments are produced from hydrogen to carbon atoms. But these got canceled due to tetrahedral geometry and the equally opposite presence of similar atoms.
So, the CH4 molecule is non-polar in nature.
The Bond Angle Of CH4:
According to VSEPR theory, the bonded atoms in a regular tetrahedral structure must spread at an angle of 109.5o. It is done to minimize the repulsion between these atoms and to make the molecule stable.
Read More: Globe HomeSurf199 Promo: 30GB Internet Data For 7 Days
So, the bond angle of CH4 is 109.5o.
Valence Electrons In CH4:
The electrons which take part in bond formation are called valence electrons. This number of electrons also depends upon the number of atoms contributing to the molecule formation.
As in the case of CH4, there is a total of 5 atoms. Four hydrogen atoms and one central carbon atom.
We can calculate the total valence electrons of CH4 by adding the valence electrons of hydrogen and carbon as follows:
Valence electrons of CH4= valence electrons of carbon + valence electrons of hydrogen.
Carbon has 4 valence electrons and each of the four hydrogen atoms contains one valence electron. If we add up all the numbers then we get the total number of CH4 valence electrons.
Valence electrons of CH4= 4 + 4 (1+1+1+1) = 8.
Where Can You Find Methane?
Methane can be found underground in the form of fossil fuel. It’s also present in wetlands, marshes, etc. as a result of decomposing organic matter.
Read More: Best Fairy Comments For Tiktok
Is Methane Harmful To Humans And The Earth?
Methane itself is not harmful but it combines with other atmospheric gases to form harmful chemicals. These chemicals may be harmful to humans. These can cause dizziness and if inhaled for a long time may cause serious consequences.
If we consider the effect of methane on the earth or atmosphere. Here it’s harmful in way that it rises the temperature by absorbing heat. Thus, causing global warming and the greenhouse effect.
Uses Of Methane (CH4):
- It is used as a fuel in different automobiles and water heaters, etc.
- It is used as an antifreeze agent in radiators and other different industries.
- It is used for sanitizing.
- It is used in combustion for the generation of electricity.
- It is also used as an ingredient in the synthesis of fertilizers.
Conclusion:
After all of the above discussion, we can conclude this article as follows:
- CH4 comprises a single central carbon atom and four hydrogen atoms preset at the four sides of the molecule.
- The molecular geometry of the CH4 molecule is tetrahedral.
- ·The hybridization of the CH4 molecule is sp3.
- The four single C-H bonds present at the four sides of the tetrahedron cancel their electronegativities of each other. This makes CH4 non-polar.
- There is a total of eight valence electrons in the CH4 molecule.
- All valence electrons are involved in bond formation so there is no loan pair present.
- The bond angle of CH4 is 109.5o.
- It has different uses in many fields of life as an important element.
Follow Techadvices.org For The Best Of The Technology.